Despite extraordinary improvements in medicine and technology over the years, the U.S. health care system often has surprisingly little access to comparative effectiveness research, or CER, which evaluates the effectiveness of medical interventions relative to each other. The Institute of Medicine, or IOM, estimates that more than half of all treatments are delivered “without clear evidence of effectiveness.” Unlike many other countries, the United States has not invested consistently in such research. For example, the U.S. Food and Drug Administration only evaluates whether new drugs are safe and work better than a placebo, not whether they are superior to other existing drugs that treat the same condition. Increased CER would arm health care consumers, providers, and payers with a greater understanding of how effective treatments truly are, which potentially would improve the quality of care and reduce health care costs.
As policymakers drafted the Affordable Care Act, or ACA, they were aware of these evidence gaps and built in a solution in the form of the private, nonprofit Patient-Centered Outcomes Research Institute, or PCORI. The ACA established PCORI with a clear mandate to carry out the “funding of comparative clinical effectiveness research” over 10 years. PCORI was to focus distinctly on CER and not duplicate the types of research funded by the Agency for Healthcare Research and Quality, the National Institutes of Health, or other entities. However, a Center for American Progress analysis in 2014 found that only one-third of PCORI’s funding was going toward CER.
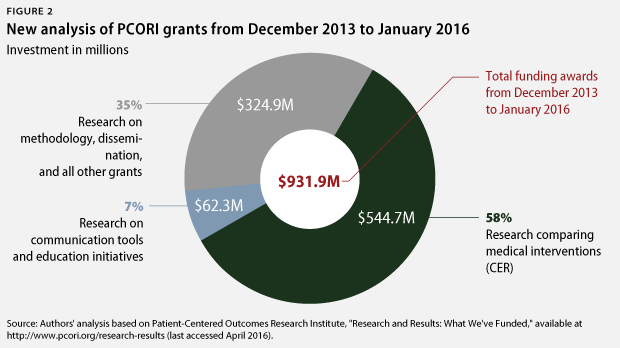
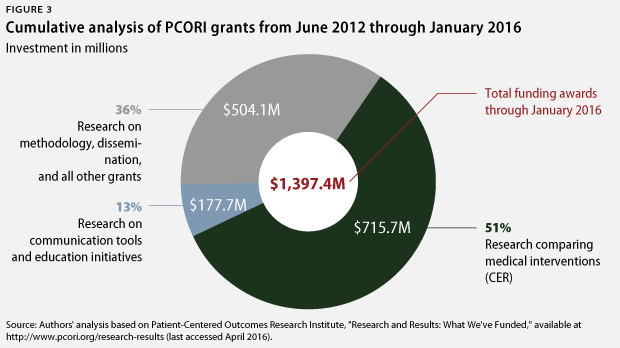
New CAP analysis of PCORI grants awarded since the 2014 report finds that PCORI has made some progress: The institute allocated 58 percent of its grant funding to CER between December 2013 and January 2016. Cumulatively through January 2016, PCORI has now dedicated 51 percent of funding to CER. However, this remains far short of CAP’s longstanding recommendation to devote at least 80 percent of funding to CER.
PCORI studies should have the potential to change clinical treatment decisions or insurers’ coverage determinations. Yet to date, this potential impact on the nation’s health care system has not been fully realized. With PCORI only authorized through 2019, the institute is running out of time to make a significant impact on CER. But if the institute acts with a new sense of urgency, it can still fulfill its role as an important tool under the ACA to reduce costs and improve the quality of care.
PCORI has made progress
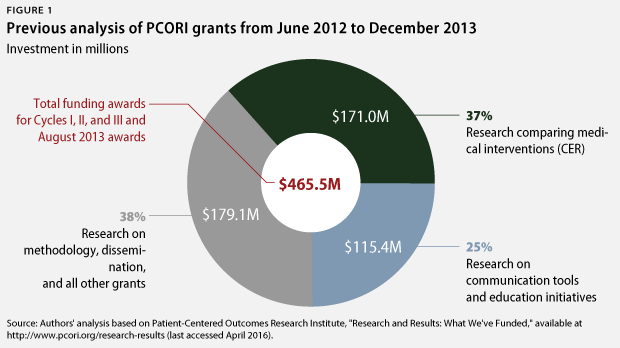
In January 2014, CAP released an evaluation of the Patient-Centered Outcomes Research Institute’s grants up to that point. CAP’s analysis found that in its first four years, PCORI allocated only 37 percent of its grant funding to comparative effectiveness research. Most of the institute’s funding had gone to studies of communication tools, patient decision aids, methodological approaches, and the establishment of data infrastructure—rather than to actual CER studies of medical interventions. Furthermore, only about 2 percent of PCORI’s grants involved prescription drugs, and none involved medical devices. Only 12 percent of grants addressed the highest-priority areas of research need identified by the Institute of Medicine. CAP recommended that PCORI increase its investment in CER to 80 percent of its funding by 2016.
The most recent CAP analysis reviewed all of PCORI’s grants to evaluate whether they funded a CER study, which was defined as comparing two or more prevention, diagnosis, or treatment alternatives. The same methodology was followed as in the 2014 assessment. CAP’s new analysis found that PCORI has increased its commitment to CER over the past two years. From December 2013 through January 2016, PCORI dedicated 58 percent of its funding to CER, compared with 37 percent in the previous analysis.
While these results fall short of CAP’s recommendation, they do represent some improvement. PCORI has shifted the distribution of its funding to focus increasingly on funding so-called pragmatic trials—which are bigger, more expensive CER trials that involve large numbers of patients in real-world settings. For instance, PCORI recently awarded $13.7 million for a study to compare two drugs for chronic obstructive pulmonary disease among 3,200 patients.
Cumulatively since its inception, PCORI has invested 51 percent of its almost $1.4 billion in grant funding in CER, totaling $716 million. PCORI has responded to CAP’s assessment, asserting that 71 percent of its cumulative funding has gone toward CER. However, PCORI’s analysis essentially just classifies all nonmethods and noninfrastructure projects as CER. Unlike PCORI, CAP researchers classify most studies of communication tools, education approaches, and patient decision-making aids in a category separate from CER. However, PCORI has substantially reduced the amount of funding going toward such studies. Such grants represented 25 percent of PCORI’s funding in CAP’s previous analysis but now only account for 13 percent of PCORI’s cumulative funding to date.
While grants for communication and education tools have decreased, PCORI has not significantly reduced the share of “other grants,” which include studies on methodology and dissemination as well as so-called engagement awards to stakeholders and academics. These engagement awards primarily fund conferences and other events. Engagement awards alone represent less than 2 percent of PCORI’s cumulative funding, but they are numerous and appear primarily to serve a public relations function.
CER focused on drugs
PCORI has made only slight progress in funding CER focused on drugs. CAP’s previous analysis found that only about 2 percent of grant funding went toward studies that involved drugs. Since December 2013, 11.3 percent of funding has gone toward CER studies involving at least one drug—but only half of this funding went toward studies that actually included head-to-head comparisons of two or more drugs. Cumulatively, only 4.4 percent of PCORI’s funding has gone toward CER comparing two or more drugs.
This funding is far too low. Of course, not all, or even most, of PCORI’s studies should focus on drugs. But prescription drugs are significantly underrepresented in PCORI’s funding. For comparison, prescription drugs represent 16.7 percent of national health spending and 19 percent of spending for Medicare and employer-sponsored health insurance plans. Thus, drug spending in the health care system is about four times the level of PCORI’s funding going toward CER studies that compare drugs.
In response to this analysis, PCORI asserts that 29 percent of studies funded in its “Assessment of Prevention, Diagnosis, and Treatment Options” category to date involved prescription drugs. However, this metric artificially inflates the percentage by using a smaller denominator—meaning that the number of studies is measured against just one of the five categories of grants, rather than the overall number of grants awarded by PCORI. Furthermore, by looking at the number of grants rather than the level of funding, PCORI’s metric says little about how many resources have actually been committed to studies of prescription drugs.
High-priority CER topics
The overall CER percentage does not show whether PCORI has focused on high-impact CER. Many of the areas where critical evidence gaps exist involve high-cost treatments, such as certain drugs, medical devices, and surgical procedures. To date, some experts and stakeholders feel that PCORI has been reluctant to engage in areas such as these where CER could generate backlash from powerful industries.
To evaluate whether PCORI’s funding was directed toward high-impact research areas, CAP examined how many of its awards addressed research questions that the Institute of Medicine identified as the top 25 highest-priority CER topics. In fairness, this is not the only relevant ranking of CER needs, and priorities may have evolved since the IOM list was published in 2009. However, the IOM list serves as an authoritative, independent rubric by which to judge whether PCORI is focusing on the most critical evidence gaps in medicine.
In its previous analysis, CAP found that only 12 percent of PCORI’s grants and 14 percent of its grant funding went toward research topics in the IOM’s top quartile. In the new CAP analysis, this first figure declined to 4 percent of grants. However, the percentage of funding allocated to the IOM topics increased to 18.5 percent. This increase indicates that, although fewer of PCORI’s grants addressed IOM priority topics, those that did address priority topics did so through larger, more expensive studies. As a result, IOM top-quartile priority topics cumulatively represent 16 percent of PCORI’s funding to date, representing relatively little change from CAP’s previous analysis.
In response to this analysis, PCORI asserts that 72 percent of the IOM’s top 25 topics have been addressed by a PCORI-funded study, along with 62 percent of the IOM’s top 100 topics. Yet this alternate framing of the issue still leaves almost 30 percent of the top quartile and almost 40 percent of the top 100 unaddressed after several years. Furthermore, this metric explains nothing about whether these studies involved significant or merely minimal funding. Many of these research topics concern broad areas of medicine where many studies are needed; as a result, CAP feels it is important not only that high-impact research topics be addressed but that a significant percentage of PCORI’s funding be connected to such topics.
New funding announcements are a step in the right direction
PCORI has already set in motion several new funding announcements that carry the potential to result in significant CER. Since these grants have not yet been awarded to specific research studies, CAP could not include them in its analysis. They should be noted, however, since these grants involve major funding in key clinical areas of need, including:
- Up to $30 million toward studies comparing approaches to managing treatment-resistant depression
- Up to $40 million toward studies comparing approaches to reducing or eliminating long-term opioid use
- Up to $30 million toward studies comparing new oral anticoagulants
- Up to $50 million toward studies comparing treatments for multiple sclerosis
- Up to $22 million toward studies comparing treatments for chronic low back pain, including spinal fusion surgery
Opioid abuse, in particular, is one of the most urgent and widespread public health crises facing the country today, and multiple sclerosis treatment is a high-cost area where major evidence gaps exist. These awards, if fully funded and dedicated to head-to-head comparison studies, are strong examples of the types of CER on which PCORI should focus.
PCORI’s current funding trajectory is not enough
The Patient-Centered Outcomes Research Institute has told CAP researchers that, going forward, it intends to dedicate 80 percent of its planned research spending to large-scale pragmatic and targeted awards. To date, CAP has considered all of these grants to be comparative effectiveness research; however, whether future pragmatic and targeted awards are CER will depend on the studies PCORI chooses to fund when scaling up the program.
According to PCORI’s projections, all of the institute’s research spending, in turn, will represent 90 percent of the total grant funds it will award from fiscal years 2016 to 2019, with the remaining 10 percent going to methodology studies, infrastructure, and engagement awards.
Using these internal funding projections, CAP estimated two possibilities for the eventual cumulative breakdown of PCORI’s funding by 2019.
In the most optimistic scenario possible under these projections, PCORI would use the entire 90 percent of its remaining funding allocated for research grants to fund CER studies. This would bring the cumulative CER measure to 69.6 percent of grant funding by 2019.
Under a more realistic projection—in which the pragmatic and targeted trials qualified as CER but the rest of the research funding exhibited the same breakdown between CER and non-CER studies as in the past—PCORI would allocate 83 percent to CER going forward. This would bring the cumulative CER measure to 66 percent of grant funding by 2019.
CAP previously recommended that PCORI dedicate at least 80 percent of its grant funding to CER by 2016, with the hopes that PCORI would build on this progress to eventually reach 80 percent CER or higher by 2019. Yet given PCORI’s funding choices to date, this cumulative goal is no longer mathematically possible.
Recommendations for a new urgency
Despite the fact that the Patient-Centered Outcomes Research Institute was explicitly created to fund comparative effectiveness research, barely half of its funding has gone to comparative effectiveness research projects. PCORI has made progress, but not enough—and it is running out of time. Since PCORI is only authorized under current law through 2019, it is urgent that the institute act quickly to maximize its impact. In its early years, PCORI made extensive investments in infrastructure, developing large research and data networks to facilitate future comparative effectiveness trials. Now, PCORI needs to take full advantage of the potential of these networks.
Going forward, PCORI must dedicate at least 90 percent of its funding awards to CER, with the goal of coming as close as possible to a cumulative 75 percent CER share. This is an aggressive target and will require reducing or eliminating PCORI’s projected spending on methods and engagement awards.
Furthermore, PCORI must focus on areas where it can make a significant and timely impact. In addition to the Institute of Medicine priorities, CAP’s definition of impact is simple: PCORI studies should have the potential to improve clinical treatment decisions or influence insurers’ coverage and reimbursement decisions.
In light of this, CAP offers five additional recommendations to improve PCORI’s impact:
- Use PCORnet, the massive data network constructed with previous PCORI funding, to track and evaluate certain new drugs as they enter the market.
- Rapidly increase investment in CER that compares drugs to a level that accurately reflects their importance to clinical practice, patient treatment, and national health spending.
- Invest more in CER for oncology, multiple sclerosis, spine surgery, and other highcost specialties where evidence gaps exist.
- Invest more in systematic reviews synthesizing existing CER studies. These can be done relatively quickly and will make it easier for providers and payers to incorporate CER studies into clinical practice and coverage decisions.
- Rank treatment alternatives by their clinical effectiveness in areas where the comparative effectiveness of different interventions has already been evaluated. This will improve the usability of CER results for patients, payers, and providers by providing a simple explanation of whether a treatment option is more or less clinically effective than its alternatives. PCORI could model this rating system after the U.S. Preventive Services Task Force’s rating system.
Thus far, PCORI has not made the impact on the health care system that the drafters of the Affordable Care Act envisioned. The need for CER remains urgent, and PCORI still has the potential to help reduce health care costs and improve the quality of care. These recommendations offer PCORI a path to maximize its impact before time runs out.
Ezekiel J. Emanuel is a Senior Fellow at the Center for American Progress. Topher Spiro is the Vice President for Health Policy at the Center. Thomas Huelskoetter is the Research Associate for Health Policy at the Center.
This publication was made possible in part by a grant from the Peter G. Peterson Foundation. The statements made and the views expressed in this issue brief are solely those of the authors.